
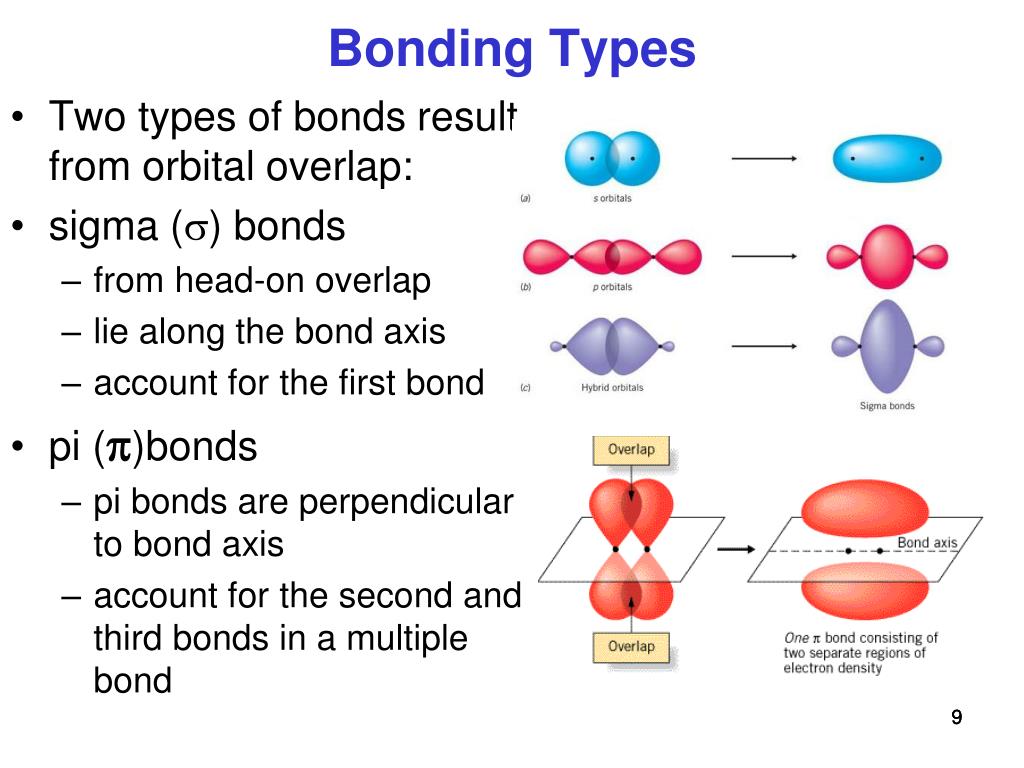
Remember that covalent bonds form from the overlap of atomic orbitals which are just the space where electrons are likely to be found.
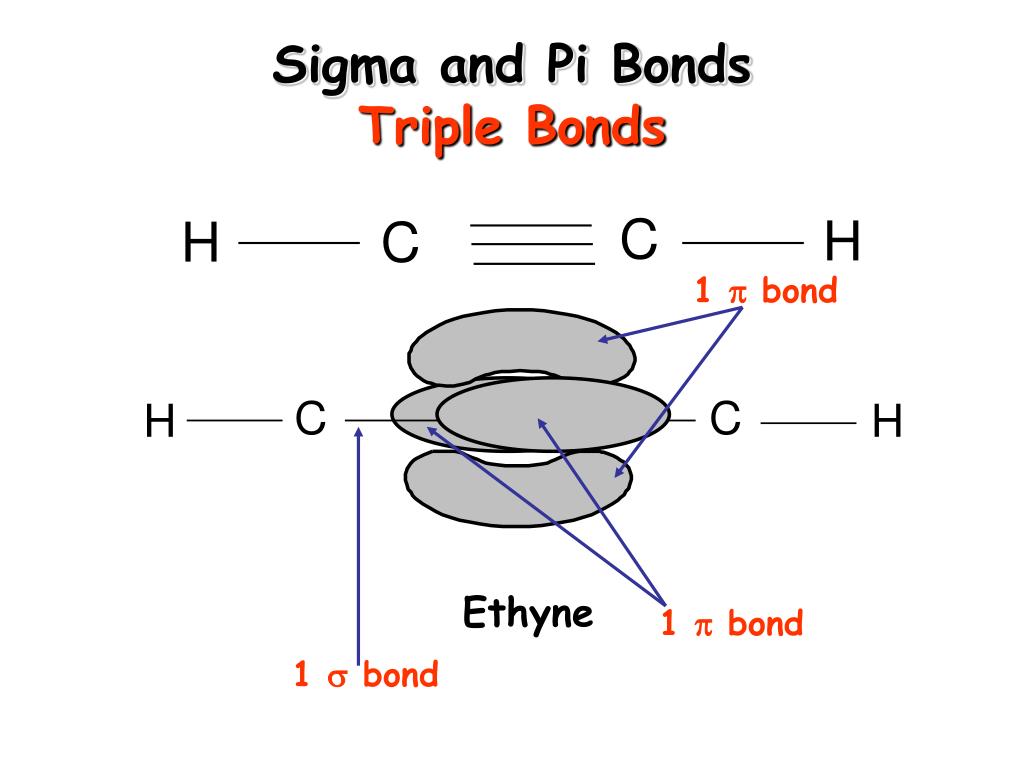
Saturated Unsaturated and Supersaturated.Reaction Quotient and Le Chatelier's Principle.Prediction of Element Properties Based on Periodic Trends.Molecular Structures of Acids and Bases.Therefore, CO2 has 2 pi bonds and 2 sigma bonds. Ion and Atom Photoelectron Spectroscopy Its px orbital will form a pi bond with the O atom that has its px orbital unhybridized, while its pz orbital will for a pi bond with the other O atoms pz orbital.Elemental Composition of Pure Substances.
 Application of Le Chatelier's Principle. Structure, Composition & Properties of Metals and Alloys. Sigma bonds form by the direct head to head overlap of atomic. Intramolecular Force and Potential Energy Sigma and pi bonds are two types of covalent bonds formed by the overlapping of atomic orbitals. Variable Oxidation State of Transition Elements. Transition Metal Ions in Aqueous Solution. Single and Double Replacement Reactions. In MO theory, a star (*) sign always indicates an anti-bonding orbital.įollowing the aufbau ('building up') principle, we place the two electrons in the H 2 molecule in the lowest energy molecular orbital, which is the (bonding) sigma orbital. The second, sigma-star ( σ *) orbital is higher in energy than the two atomic 1 s orbitals, and is referred to as an anti-bonding molecular orbital. According to MO theory, the first sigma orbital is lower in energy than either of the two isolated atomic 1 s orbitals – thus this sigma orbital is referred to as a bonding molecular orbital. When two atomic 1 s orbitals combine in the formation of H 2, the result is two molecular orbitals called sigma ( σ) orbitals. The bonding in H 2, then, is due to the formation of a new molecular orbital (MO), in which a pair of electrons is delocalized around two hydrogen nuclei.Īn important principle of quantum mechanical theory is that when orbitals combine, the number of orbitals before the combination takes place must equal the number of new orbitals that result – orbitals don’t just disappear! We saw this previously when we discussed hybrid orbitals: one s and three p orbitals make four sp 3 hybrids. These two new orbitals, instead of describing the likely location of an electron around a single nucleus, describe the location of an electron pair around two or more nuclei. In molecular orbital theory, we make a further statement: we say that the two atomic 1 s orbitals don’t just overlap, they actually combine to form two completely new orbitals. When we described the hydrogen molecule using valence bond theory, we said that the two 1 s orbitals from each atom overlap, allowing the two electrons to be shared and thus forming a covalent bond. Sigma bond formatin brings suitable orbitals into sideways. Let’s consider again the simplest possible covalent bond: the one in molecular hydrogen (H 2). Pi bonds can only form AFTER sigma bonds have already formed. A sigma bond forms when two hybridized orbitals overlap (such as sp, sp2, or sp3 hybridized orbitals) whereas pi bonds form when 2 p orbitals overlap side by side. \)Īnother look at the H 2 molecule: bonding and anti-bonding sigma molecular orbitals
Application of Le Chatelier's Principle. Structure, Composition & Properties of Metals and Alloys. Sigma bonds form by the direct head to head overlap of atomic. Intramolecular Force and Potential Energy Sigma and pi bonds are two types of covalent bonds formed by the overlapping of atomic orbitals. Variable Oxidation State of Transition Elements. Transition Metal Ions in Aqueous Solution. Single and Double Replacement Reactions. In MO theory, a star (*) sign always indicates an anti-bonding orbital.įollowing the aufbau ('building up') principle, we place the two electrons in the H 2 molecule in the lowest energy molecular orbital, which is the (bonding) sigma orbital. The second, sigma-star ( σ *) orbital is higher in energy than the two atomic 1 s orbitals, and is referred to as an anti-bonding molecular orbital. According to MO theory, the first sigma orbital is lower in energy than either of the two isolated atomic 1 s orbitals – thus this sigma orbital is referred to as a bonding molecular orbital. When two atomic 1 s orbitals combine in the formation of H 2, the result is two molecular orbitals called sigma ( σ) orbitals. The bonding in H 2, then, is due to the formation of a new molecular orbital (MO), in which a pair of electrons is delocalized around two hydrogen nuclei.Īn important principle of quantum mechanical theory is that when orbitals combine, the number of orbitals before the combination takes place must equal the number of new orbitals that result – orbitals don’t just disappear! We saw this previously when we discussed hybrid orbitals: one s and three p orbitals make four sp 3 hybrids. These two new orbitals, instead of describing the likely location of an electron around a single nucleus, describe the location of an electron pair around two or more nuclei. In molecular orbital theory, we make a further statement: we say that the two atomic 1 s orbitals don’t just overlap, they actually combine to form two completely new orbitals. When we described the hydrogen molecule using valence bond theory, we said that the two 1 s orbitals from each atom overlap, allowing the two electrons to be shared and thus forming a covalent bond. Sigma bond formatin brings suitable orbitals into sideways. Let’s consider again the simplest possible covalent bond: the one in molecular hydrogen (H 2). Pi bonds can only form AFTER sigma bonds have already formed. A sigma bond forms when two hybridized orbitals overlap (such as sp, sp2, or sp3 hybridized orbitals) whereas pi bonds form when 2 p orbitals overlap side by side. \)Īnother look at the H 2 molecule: bonding and anti-bonding sigma molecular orbitals







 0 kommentar(er)
0 kommentar(er)
